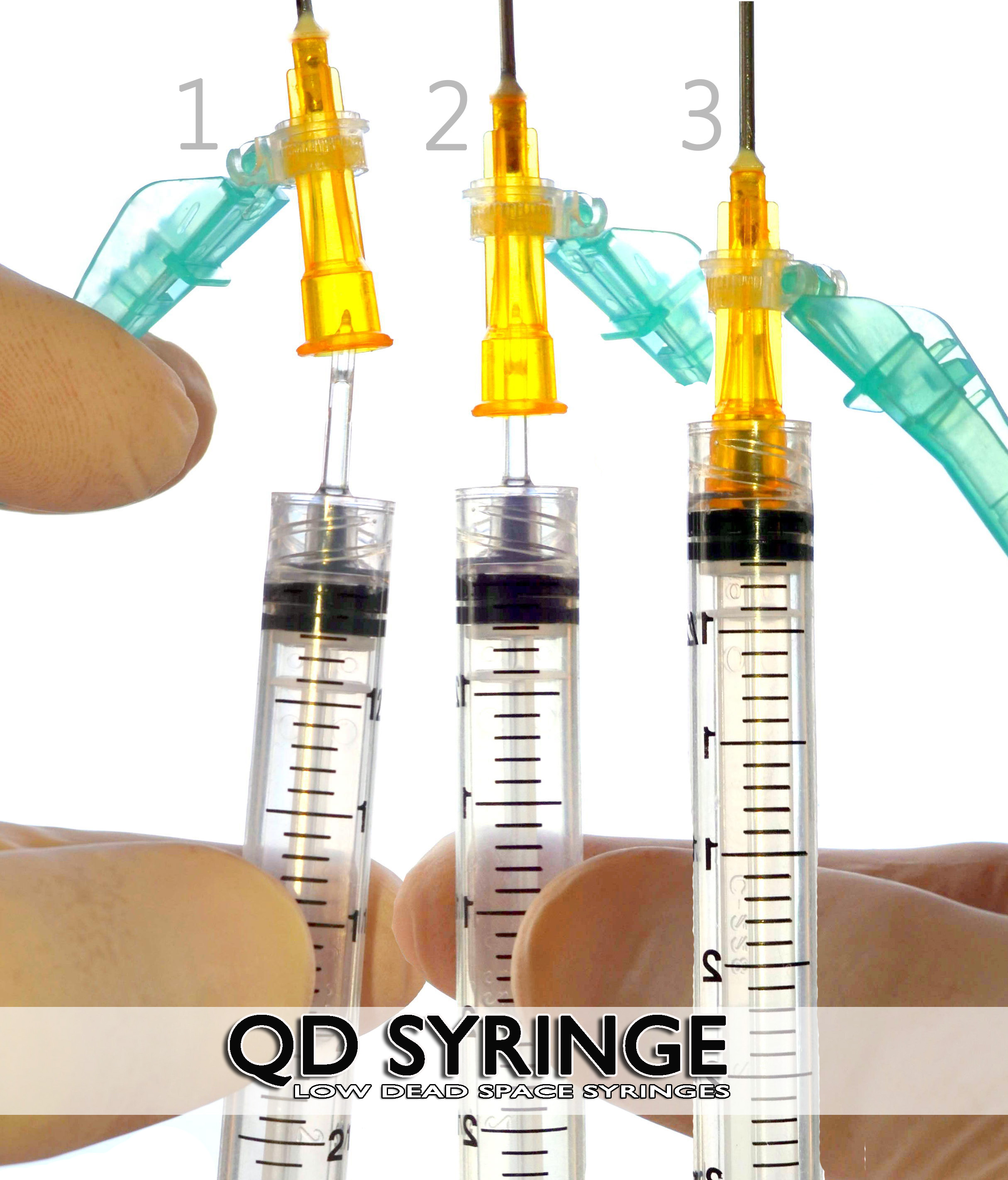
QD Syringe – QD Hubs and Needles
QD Syringes are the next generation of basic disposable plastic syringes with detachable injection hubs with needles. The QD Syringe does not need a detachable draw needle to draw fluid inside of its barrel. The QD Syringe has an integrated BPA free acrylic tip with GlyFlo Technology to accomplish this task. The QD Syringe is a low dead space syringe and has the sharpest needles of any injection syringe available.
The QD Syringe works by being functional right out of it’s package. It is the only syringe that is able to draw fluid into itself. Then the appropriate steel needle gauge and length is attached over it’s tip and a virtually painless injection is given.
The painless injection is accomplished by not using the sharp steel needle to draw up medications and dulling its tip prior to giving the patient injection. The QD Syringe gives IV push injections and is also compatible with a majority of pre-slit injection receptacles such as the BD Q-Syte. All of this is accomplished by the low dead space QD Syringe. The QD Syringe is a BPA Free Medical Syringe. Coming Soon: The QD Neutral Displacement Needleless Connector which will be compatible with the QD Syringe and standard luer lock syringes.